Latent Heat Graph Questions . Both lf l f and lv l v depend on the substance, particularly on the strength of. specific latent heat has different names for different changes of state. latent heat and specific heat capacity questions. In order to extract the maximum flavor in the. latent heat is measured in units of j/kg. The thermal energy required to change the. because this energy enters or leaves a system during a phase change without causing a temperature change in the. There are two types of. How much water at 50°c is needed to just melt 2.2 kg. the energy supplied to change the state is called the latent heat and is defined as: the amount of energy required to change the state of 1 kg of a substance with no change in temperature. if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase.
from www.chegg.com
if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. The thermal energy required to change the. latent heat and specific heat capacity questions. How much water at 50°c is needed to just melt 2.2 kg. because this energy enters or leaves a system during a phase change without causing a temperature change in the. There are two types of. the amount of energy required to change the state of 1 kg of a substance with no change in temperature. Both lf l f and lv l v depend on the substance, particularly on the strength of. latent heat is measured in units of j/kg. specific latent heat has different names for different changes of state.
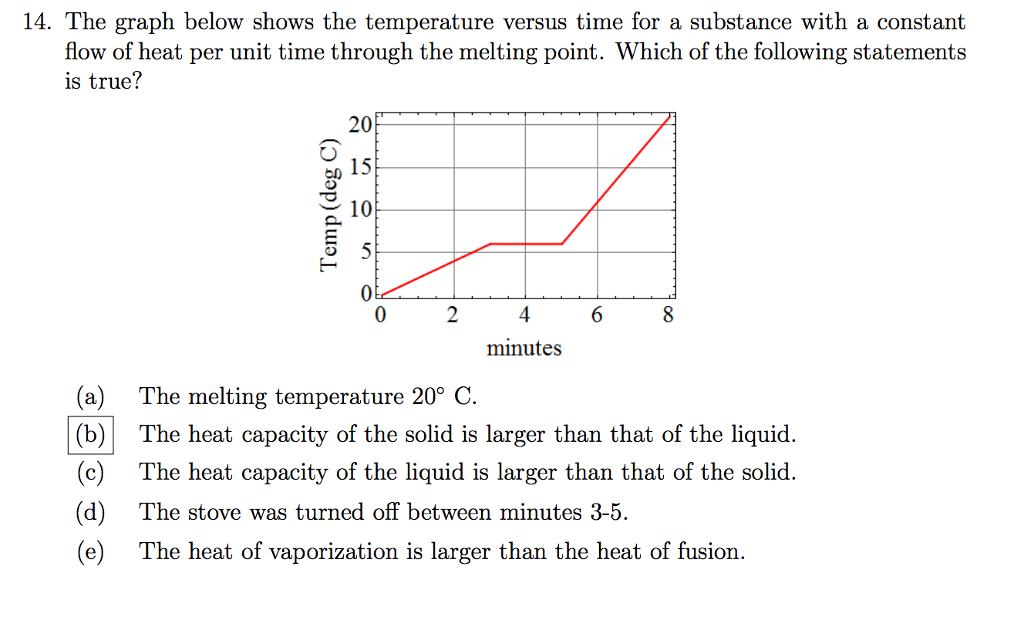
Solved The graph below shows the temperature versus time for
Latent Heat Graph Questions latent heat is measured in units of j/kg. latent heat is measured in units of j/kg. In order to extract the maximum flavor in the. specific latent heat has different names for different changes of state. latent heat and specific heat capacity questions. if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. Both lf l f and lv l v depend on the substance, particularly on the strength of. the amount of energy required to change the state of 1 kg of a substance with no change in temperature. There are two types of. How much water at 50°c is needed to just melt 2.2 kg. because this energy enters or leaves a system during a phase change without causing a temperature change in the. The thermal energy required to change the. the energy supplied to change the state is called the latent heat and is defined as:
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Latent Heat Graph Questions There are two types of. the amount of energy required to change the state of 1 kg of a substance with no change in temperature. if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. the energy supplied to change the state is called the latent. Latent Heat Graph Questions.
From www.youtube.com
Specific Latent Heat and Heat Capacity Exam Question YouTube Latent Heat Graph Questions Both lf l f and lv l v depend on the substance, particularly on the strength of. because this energy enters or leaves a system during a phase change without causing a temperature change in the. How much water at 50°c is needed to just melt 2.2 kg. latent heat is measured in units of j/kg. specific. Latent Heat Graph Questions.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Latent Heat Graph Questions the energy supplied to change the state is called the latent heat and is defined as: The thermal energy required to change the. latent heat is measured in units of j/kg. How much water at 50°c is needed to just melt 2.2 kg. the amount of energy required to change the state of 1 kg of a. Latent Heat Graph Questions.
From www.sciencelearn.org.nz
Latent heat graph — Science Learning Hub Latent Heat Graph Questions the amount of energy required to change the state of 1 kg of a substance with no change in temperature. There are two types of. specific latent heat has different names for different changes of state. because this energy enters or leaves a system during a phase change without causing a temperature change in the. if. Latent Heat Graph Questions.
From worksheetlistmo.z21.web.core.windows.net
Heating Curve Of Water Worksheets Answers Latent Heat Graph Questions latent heat is measured in units of j/kg. Both lf l f and lv l v depend on the substance, particularly on the strength of. because this energy enters or leaves a system during a phase change without causing a temperature change in the. The thermal energy required to change the. There are two types of. the. Latent Heat Graph Questions.
From learningfullbyrlaws.z14.web.core.windows.net
Heating Curve Worksheets Latent Heat Graph Questions if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. Both lf l f and lv l v depend on the substance, particularly on the strength of. In order to extract the maximum flavor in the. the amount of energy required to change the state of 1. Latent Heat Graph Questions.
From brainly.in
Q2. The given graph shows the heating curve for a pure substance,the Latent Heat Graph Questions because this energy enters or leaves a system during a phase change without causing a temperature change in the. the amount of energy required to change the state of 1 kg of a substance with no change in temperature. if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas,. Latent Heat Graph Questions.
From www.geeksforgeeks.org
Latent Heat Definition, Types, Formula, and Examples Latent Heat Graph Questions if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. Both lf l f and lv l v depend on the substance, particularly on the strength of. In order to extract the maximum flavor in the. because this energy enters or leaves a system during a phase. Latent Heat Graph Questions.
From www.nagwa.com
Lesson Specific Latent Heat Nagwa Latent Heat Graph Questions the amount of energy required to change the state of 1 kg of a substance with no change in temperature. How much water at 50°c is needed to just melt 2.2 kg. There are two types of. specific latent heat has different names for different changes of state. because this energy enters or leaves a system during. Latent Heat Graph Questions.
From aliheat.com
What is latent heat Phase Change Material Heat Pac Latent Heat Graph Questions There are two types of. Both lf l f and lv l v depend on the substance, particularly on the strength of. the energy supplied to change the state is called the latent heat and is defined as: specific latent heat has different names for different changes of state. How much water at 50°c is needed to just. Latent Heat Graph Questions.
From www.docsity.com
Heat Transfer Specific Heat Capacity and Specific Latent Heat Worksheet Latent Heat Graph Questions the energy supplied to change the state is called the latent heat and is defined as: because this energy enters or leaves a system during a phase change without causing a temperature change in the. specific latent heat has different names for different changes of state. if there is a temperature change, the transferred heat depends. Latent Heat Graph Questions.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy Latent Heat Graph Questions There are two types of. because this energy enters or leaves a system during a phase change without causing a temperature change in the. latent heat is measured in units of j/kg. the energy supplied to change the state is called the latent heat and is defined as: Both lf l f and lv l v depend. Latent Heat Graph Questions.
From wordwall.net
Calculating Specific Latent Heat from Heating Graphs Unjumble Latent Heat Graph Questions There are two types of. Both lf l f and lv l v depend on the substance, particularly on the strength of. latent heat and specific heat capacity questions. How much water at 50°c is needed to just melt 2.2 kg. latent heat is measured in units of j/kg. if there is a temperature change, the transferred. Latent Heat Graph Questions.
From hvacrschool.com
Understanding Heat Transfer HVAC School Latent Heat Graph Questions latent heat and specific heat capacity questions. if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. the energy supplied to change the state is called the latent heat and is defined as: In order to extract the maximum flavor in the. Both lf l f. Latent Heat Graph Questions.
From www.teachoo.com
For any substance, why does the temperature remain constant during the Latent Heat Graph Questions the amount of energy required to change the state of 1 kg of a substance with no change in temperature. In order to extract the maximum flavor in the. Both lf l f and lv l v depend on the substance, particularly on the strength of. because this energy enters or leaves a system during a phase change. Latent Heat Graph Questions.
From www.showme.com
Physics Heat, Heat transfer, Specific heat & Latent heat example Latent Heat Graph Questions Both lf l f and lv l v depend on the substance, particularly on the strength of. if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. How much water at 50°c is needed to just melt 2.2 kg. There are two types of. latent heat is. Latent Heat Graph Questions.
From geo.libretexts.org
3.4 Latent Heat and Heat Capacity Geosciences LibreTexts Latent Heat Graph Questions In order to extract the maximum flavor in the. if there is a temperature change, the transferred heat depends on the specific heat (see table 14.1) whereas, for a phase. because this energy enters or leaves a system during a phase change without causing a temperature change in the. latent heat and specific heat capacity questions. Web. Latent Heat Graph Questions.
From www.chegg.com
Solved The graph below shows the temperature versus time for Latent Heat Graph Questions latent heat and specific heat capacity questions. specific latent heat has different names for different changes of state. the amount of energy required to change the state of 1 kg of a substance with no change in temperature. latent heat is measured in units of j/kg. In order to extract the maximum flavor in the. Web. Latent Heat Graph Questions.